Antenatal Steroids for Improving Survival of Preterm Babies
Why Preterm Birth Is Important
Every year, approximately 15 million babies are born too soon, and an estimated 1 million babies die due to complications associated with prematurity. The situation is significantly worse in low-income countries, where half of all babies born at or below 32 weeks die due to a lack of feasible lifesaving interventions.
For more than four decades, the use of antenatal corticosteroids (e.g., dexamethasone) has been known to improve fetal lung maturity and reduce subsequent respiratory morbidity and death in preterm infants. Despite this knowledge, a substantial proportion of premature babies in low- and middle-income countries (LMICs) die needlessly, in part due to poor coverage of antenatal corticosteroids.
But Preterm Infant Survival Is Not Just about Antenatal Steroids Coverage

Photo Courtesy UNICEF/Asselin
For several decades, the global community viewed antenatal steroids as the magic bullet to reduce the high burden of preterm infant and under 5 mortality in low-resource settings. Toward the end of the Millennium Development Goal era, the focus was to scale up the use of antenatal steroids everywhere through any means possible. Skeptics warned at the time against indiscriminate use of antenatal steroids, citing the double-edged nature of steroids on the human body; and proponents advocated for the universal use of antenatal steroids not just for babies at risk of being born preterm (<37 weeks) but also for some babies born at term!
An attempt to demonstrate the benefits of scale-up of antenatal steroids in six LMICs (Argentina, Guatemala, India, Kenya, Pakistan and Zambia) unexpectedly produced negative results that shocked the world—no effect of scale-up of antenatal steroids on neonatal death among small (preterm) babies, but instead more neonatal deaths and infections in the mothers at the population level. This antenatal corticosteroids trial (ACT) made us think that the skeptics might be right: Could it be that the effects of steroids in high-income settings have been overestimated, that the overall quality of care in LMICs is insufficient to counteract the potential negative effects of steroids, or that there are other determinants of preterm infant death in LMICs that steroids could not address? There were more questions than answers and the global drive for scale-up of antenatal steroids in LMICs literally came to a grinding halt.
The World Health Organization (WHO) in its 2015 guidelines decided to balance the evidence of antenatal steroid efficacy largely derived from high-income settings with the safety concerns from LMICs by recommending antenatal steroids only when a certain level of quality of care can be made available to both mother and preterm infant. It became clear that the question of the efficacy and safety of antenatal steroids in low-resource countries needed to be revisited.
From ACT to ACTION
The safety concerns about antenatal steroids from ACT led to calls for new studies in a more controlled research settings in low-resource countries. WHO responded to this call by coordinating a clinical trial that recruited 2,852 women and their 3,070 babies from 29 hospitals in Bangladesh, India, Kenya, Nigeria and Pakistan between December 2017 and November 2019. The results of this clinical trial, published in the New England Journal of Medicine, show that dexamethasone—a steroid used to treat many conditions, including rheumatic problems—can boost survival of premature babies when given to pregnant women at risk of early preterm birth (between 26 and 34 weeks) in low-resource settings.
The WHO-led Antenatal Corticosteroid for Improving Outcomes in Preterm Newborns (ACTION) trial resolves an ongoing controversy about the efficacy of antenatal steroids for improving preterm infant survival in low-resource countries. This is the first time a clinical trial has proven that dexamethasone is also effective in low-income settings—a huge sigh of relief that the world would not have to do away with a “good drug” for reducing preterm newborn mortality in resource-limited settings, and Cochrane Collaboration can keep its logo, which was coined from meta-analysis of studies of antenatal steroids!
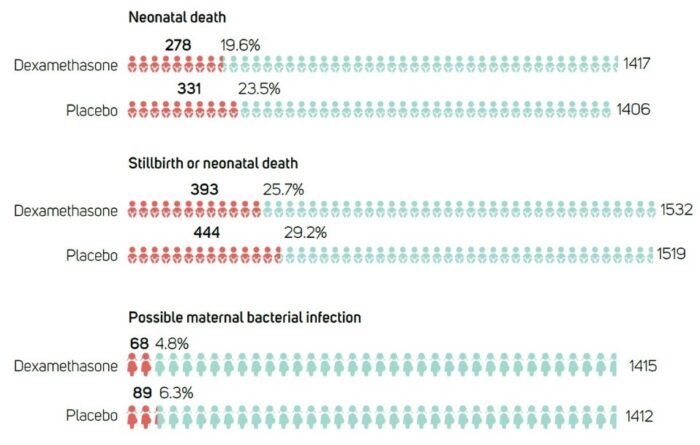
As Figure 1 shows, the ACTION trial’s impact is significant: for every 25 pregnant women treated with dexamethasone, one premature baby’s life was saved. The study also found a significantly lower risk of any baby death (neonatal death and stillbirth), and no increase in maternal bacterial infections with dexamethasone. It should be clear from these results that dexamethasone is now a proven drug to save babies born too soon in low-income settings. However, no one should lose sight of the fact that it is only effective when administered by health care providers who can make timely and accurate decisions about gestational age and high probability of preterm birth, and provide a minimum package of high-quality care for both pregnant women and their babies.
The study notes that health care providers must have the means to select the women most likely to benefit from the drug and to correctly initiate the treatment at the right time—that is, the right treatment for the right population of pregnant women at the right time.
What Now?
Getting clarity on the efficacy of antenatal steroids in reducing preterm newborn mortality in low-resource countries is just the start. Selection of pregnant women whose babies are most likely to benefit from antenatal steroids in LMICs as suggested by the ACTION trial is not straightforward. Accurate diagnosis of preterm labor is by default challenging even by specialists and is likely to be problematic in health care settings where specialists are few. Early gestational age dating is not yet universal as many women in low-resource settings in sub-Saharan Africa and South Asia tend to start antenatal care late when ultrasound dating is less reliable. We still don’t know if antenatal steroids are beneficial for late preterm babies in low-resource countries and whether the currently recommended dose of antenatal steroids is too high.
Nonetheless, the end is in sight, and we should celebrate the progress made so far. The natural next step for the global community is to identify effective strategies to scale up the safe use of antenatal steroids in a way that maximizes benefits and minimizes risk for the mother and preterm infant at both hospital and population levels in LMICs.
With the growing innovations around low-cost ultrasound technologies and devices, as well as artificial intelligence-driven patient-selection techniques, there is hope for the world’s poorest countries to fast-track progress toward attaining relevant targets of the 2030 agenda for sustainable development.
Professor Femi Oladapo, Head, Maternal and Perinatal Health Unit, UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization
