Q&A: Implementation Considerations of New WHO Recommendations for Postpartum Hemorrhage
Postpartum hemorrhage, or PPH—defined as blood loss of 500 mL or more within 24 hours after birth—is the leading cause of maternal death worldwide. Every year, PPH results in around 70,000 deaths, with most occurring in sub-Saharan Africa and South Asia. It is imperative that we address the urgent issue of PPH in order to get back on track towards the Sustainable Development Goal (SDG) target for reducing maternal mortality.
In December 2023, the World Health Organization (WHO) issued new recommendations on the assessment of postpartum hemorrhage (PPH) and a new recommendation on the use of a treatment bundle for the management of PPH. These new recommendations came on the heels of published evidence from the E-MOTIVE trial, which tested a bundle of care approach to PPH management.
The PPH Community of Practice, hosted by USAID’s MOMENTUM Country and Global Leadership, held a webinar in March 2024 to discuss the implementation considerations and lessons learned related to these new recommendations on PPH from WHO. Below are a set of key questions we have received about implementing the recommendations and the bundle of care approach, along with our responses.
Clinical Bundles and E-MOTIVE
Q: What is a clinical bundle?
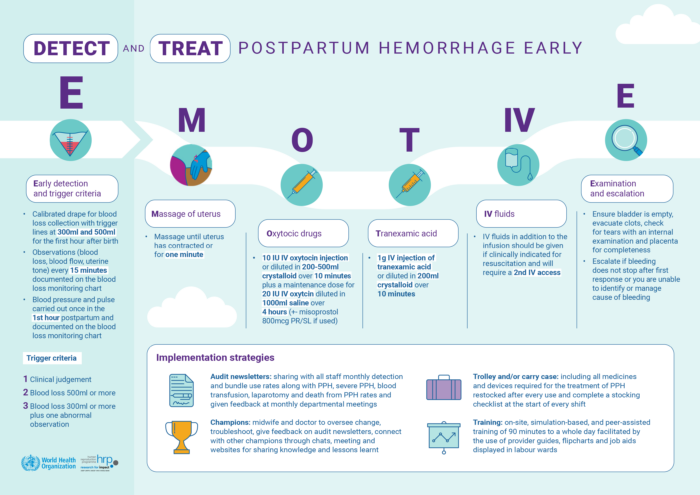
A: Clinical care bundles are a structured way of improving the processes of care and patient outcomes. They include administration of a set of evidence-based practices — generally three to five — that, when performed collectively and reliably as soon as a condition is diagnosed or “triggered”, have been demonstrated to improve patient outcomes. The important element is that all women should receive all components of the 1st response bundle for postpartum hemorrhage (PPH) at the same time irrespective if bleeding settles.
Q: Is E-MOTIVE the same as the WHO 1st response bundle for PPH?
A: The first “E” in E-MOTIVE is for early detection. The bundle approach was originally conceptualized and led by WHO in a technical consultation. This was adapted for use in the EMOTIVE trial and constitutes the “MOTIVE” bundle. All components of the bundle were already individually recommended by WHO in existing guidelines.
Q: What is in the bundle and who can give it?
A: The components are:
- Massage
- Oxytocic (uterotonic)
- Tranexamic acid
- IV access and crystalloid fluids (not colloids)
- Examination & Escalation
All components given as soon as possible by skilled health care providers and within 15 minutes of diagnosis of PPH. One of the important implementation strategies in E-MOTIVE was the development of clinical protocols to enable midwives to be able to give all bundle components independently.
All facilities that offer maternity services should be able to provide all components of the bundle. In lower-level facilities, escalation may include referral for higher-level of care.
Q: How do you manage the “E” for escalation if you are not in a referral center?
A: Escalation means moving on to refractory treatment as soon as the bundle is seen to have failed. For lower-level facilities, it is important to consider at which point referral to a higher-level facility is appropriate.
While activating the referral plan, lower-level sites should consider what refractory care they can as rapidly as they can, which may include additional uterotonics, NASG, bimanual uterine or aortic compression.
Q: It would be great to hear more about the simulation/debrief component.
A: For the E-MOTIVE trial and the global version of the PPH training materials, learning included simulation in small groups for participants to practice and review their performance after completing the simulated case.
PPH Medications and Measurement Tools
Q: What is the purpose of drapes? Will the World Health Organization (WHO) make policy briefs for all countries to acquire drapes? How do you use drapes? Where do you get drapes?
A: Currently the guideline recommends objective blood loss assessment, this may be drapes but other tools can be used too for this purpose. The recommendation clarifies that most of the evidence applies to calibrated drapes but appreciates they are not the only devices available for this purpose. Hence, WHO would not be issuing policy briefs encouraging countries to purchase a specific device for objective measurement of blood loss.
The drape can be folded behind the woman and tied loosely around her waist in late second stage, so she is able to move and deliver in the position of her choice. After birth and administration of a uterotonic within one minute, unroll the drape or place the blood measuring tool before cutting the cord. Maintain the drape/tool for the first hour after birth, observing the blood loss against the calibration markings.
Q: Can you use heat-stable carbetocin (HSC) instead of oxytocin in the bundle?
A: No, HSC is neither licensed nor recommended for use in treatment of PPH, only for prevention of PPH. Research is currently underway to establish the safety and efficacy of HSC for treatment of PPH in the context where HSC is already in use for prevention of PPH through the REACH trial. Due to the mechanism and length of action, HSC should also NEVER be used for augmentation and induction.
Q: Is tranexamic acid (TXA) for prevention or treatment? Does it replace other treatments such as bimanual?
A: TXA is used as a treatment and is one component of the bundle for all women with PPH. Studies are underway to evaluate efficacy and safety for prevention. TXA does not replace any other treatments in the bundle. Bimanual uterine compression can be used – along with other required treatments – if the bundle fails and refractory treatment is needed.
Q: Could the use of tranexamic acid in lower facilities as part of the bundle delivered within 15 minutes potentially risk overdose?
A: TXA is a very safe drug and the recommended dose of 1g given IV over 10 minutes is safe to repeat 30 minutes after the first dose if the woman is still bleeding OR begins bleeding again within 24 hours.
Dissemination and Implementation
Q: What are the next steps for WHO?
A: WHO is working towards implementation of the PPH roadmap. A key pillar within the roadmap is the development of a consolidated guideline for PPH detection, prevention and management including adoption of early detection and a bundle approach which will be a one-stop-shop of clinical guidance and recommendations to enhance and accelerate improvements in PPH care.
Q: Can we have access to training materials for the bundle?
A: Training materials are being adapted to reflect upcoming consolidated guidelines including prevention, detection, and treatment using the first response bundle and refractory care.
Jhpiego and Laerdal Foundation have committed to work with WHO in producing implementation materials including training materials to implement the full suite of WHO PPH recommendations. We look forward to sharing this work with the Postpartum Hemorrhage Community of Practice in the future. A link to current training materials which include objective measurement and the first response bundle is here.
Q: How do we ensure that women coming from the community who are bleeding receive the treatment bundle?
A: For women coming from the community or referred for PPH from a lower-level facility, treatment with the bundle should begin immediately upon arrival. Tranexamic acid (TXA) can be used if the birth was within three hours. If the birth was more than three hours, it should not be used but other components should be given as rapidly as possible.
Q: How do you institutionalize the system shift to both measurement of blood and the quick and complete response?
A: As part of the recommendation development process, implementation considerations were developed. These may assist policymakers, clinicians and other stakeholders to better prepare for implementation. Details of these considerations can be found in the guideline Implementation considerations section.
Q: What are some implementation strategies?
A: These are described well in the Randomized Trial of Early Detection and Treatment of Postpartum Hemorrhage | New England Journal of Medicine paper and include:
- Onsite training – simulation and team based
- Champions – facility based in-charges or facility heads need to be brought on board
- Trolleys – Designed to ensure everything needed is in one place.
- Audit and Feedback – This was a newsletter given to sites telling them the numbers and proportions of PPH cases and the numbers and proportions of those cases that were treated in order to let them know how they were doing.
The Postpartum Hemorrhage Community of Practice (PPH CoP) is a dynamic, interconnected, global learning platform that supports the dissemination and implementation of current evidence, and the sharing of country-to-country experiences to achieve global targets for postpartum hemorrhage. To join the PPH CoP, please fill out this form. For general inquiries, please contact pph.cop@jhpiego.org.