Postpartum Hemorrhage
Global Recommendations and Guidance
This page includes a select set of WHO global recommendations and guidance on PPH.
The World Health Organization has published a number of important recommendations related to Postpartum Hemorrhage (PPH) within the last 10 years.
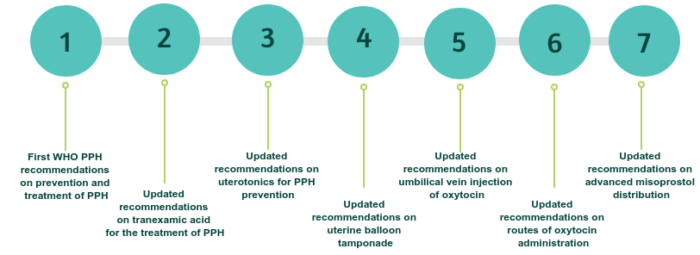
In 2012, WHO published the first PPH recommendations on prevention and treatment of PPH.
In 2017, in response to new evidence (WOMAN trial), WHO updated the recommendation on tranexamic acid for PPH treatment, with a supporting briefer which outlines highlights and key messages from this updated recommendation.
In 2018, in light of the new evidence (WHO CHAMPION trial), the WHO recommendations on uterotonics for PPH prevention were updated.
In 2020, four recommendations were updated:
1) WHO recommendation on Uterine balloon tamponade for the treatment of postpartum haemorrhage
2) WHO recommendation on umbilical vein injection of oxytocin for the treatment of retained placenta
3) WHO recommendation on routes of oxytocin administration for the prevention of postpartum haemorrhage after vaginal birth
4) WHO recommendation on Advanced misoprostol distribution to pregnant women for prevention of postpartum hemorrhage.
In 2023, WHO and HRP published the Roadmap to combat postpartum hemorrhage between 2023-2030. This publication outlines goals and activities in four strategic areas necessary to accelerate progress: research, norms and standards, implementation, and advocacy.
In 2023, the World Health Organization (WHO) also convened a Guideline Development Group to update an existing recommendation on assessing postpartum hemorrhage (PPH) and consider using a care bundle to treat PPH. This decision was based on new evidence on the subject that had become available. The guidelines issued an updated recommendation on the assessment of PPH and a new recommendation on the use of a treatment bundle for the management of PPH.

Model List of Essential Medicines, 21st List
World Health Organization, 2019.
The 2019 core list from WHO presents the minimum medicine needs for a basic health-care system, listing the most efficacious, safe and cost–effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost-effective treatment. Uterotonics are presented on pg. 47.

Protecting the Blood Supply During Infectious Disease Outbreaks: Guidelines for National Blood Services
World Health Organization, 2019.
This 2019 guidance document has been produced by WHO to assist blood services in the development of national plans to respond to any emerging infectious threats to the sufficiency or safety of the blood supply, whether from an existing infectious agent that is changing in incidence and spread, or from a newly identified infectious agent.

